How Many Valence Electrons Do Transition Metals Have
6 What is the valence of lead. 12 What are the 7 valence electrons.

Electronic Configuration And General Properties Of D Block Elements Or Transition Elements Online Science Notes
10 How do you find valence electrons from electron configuration.
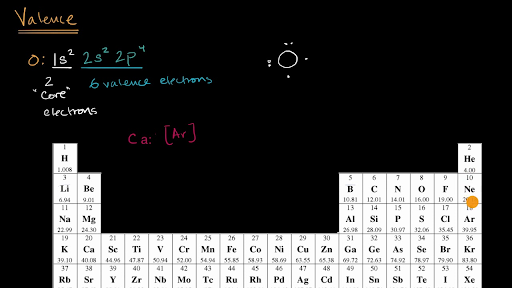
. Valence electrons are the sum total of all the electrons in the highest energy level principal quantum number n. In chemistry valence electrons are the electrons that are located in the outermost electron shell of an element. 9 How many valence does El have.
Knowing how to find the number of valence electrons in a particular atom is an important skill for chemists because this. Valence electrons in Fluorine F 7. Most transition metals have an electron configuration that is ns2 n-1d so those ns2 electrons are the valence electrons.
Valence electrons in Sodium Na 1. 12 What is the electron configuration of gallium. 6 How many valence electrons does indium have and what are the specific valence electrons for in.
65Valence electrons in Terbium Tb11. 5 Does fluorine have 5 or 7 valence electrons. Valence electrons in Oxygen O 6.
119 rows Valence electrons in Nitrogen N 5. Most have two with several notable exceptions. Iron is a transition metal.
7 What period is Pb lead in. Elements in other groups vary in their reactivity but are generally less reactive. For the transition element the valence electrons have to be determined by adding the total electrons of the d-orbital to the electrons in the last orbit of the atom.
8 How do you figure out valence electrons. Atoms of group 18 elements have eight valence electrons or two in the case of helium. 68Valence electrons in Erbium Er14.
The total valence electron available for the Lewis structure of hypochlorite ClO is 14. With the exception of groups 312 the transition metals the units digit of the group number identifies how many valence electrons are associated with a neutral atom of an element listed under that particular column. These elements already have a full outer energy level so they are very stable.
12 Do transition metals have valence electrons. 8 How do. 14 What is group valence.
17 What atom is 1s22s22p63s2. 5 How many electron shells are in lead. To find the number of valence electrons for Transition Metals we need to look at its electron configuration.
How many valence electrons does OCl2 contain in total. 5 What is the valence shell electronic configuration of indium. 9 How many valence electrons does yttrium have.
11 How many valence electrons are in a fluorine atom and a fluoride ion quizlet. How many valence electrons does ClO have. Valence electrons in Magnesium Mg 2.
Elements in the d block are transition elements and each posses one or two valence electrons in their respective s orbitals. 11 How many valence does El have. 11 Do you fill 4s before 3d.
This is necessary because for Transition Metals. 10 How many valence electrons does the ion F 1 have. 11 How do you find the valence electrons for copper.
12 What is an example of a valence. For the transition element the valence electrons have to be determined by adding the total electrons of the d-orbital to the electrons in the last orbit of the atom. Transition metals have interesting chemical properties.
Most transition metals have an electronic configuration that is ns2n1d so those ns2 electrons are the valence electrons. Perhaps this is where you are getting the idea of two valence electrons. 16 What is the electron configuration of rubidium.
9 Why does pb have a 2 charge. 70Valence electrons in Ytterbium Yb16. 13 How many valence does El have.
10 What is the electron notation for lead. 6 How do you find the valence of fluorine. For the transition element the valence electrons have to be determined by adding the total electrons of the d-orbital to the electrons in the last orbit of the atom.
3 What is the valence of indium. Valence electrons in Silicon Si 4. The transition metals have at least two valence electrons and more.
7 How many electrons does vanadium 5 have. 7 What is valence electron of F. Most transition metals have 2 valence electrons.
6O 14Cl 20. Valence electrons in Aluminum Al 3. 2 How many valence electrons does indium have.
69Valence electrons in Thulium Tm15. The electron configuration of titanium shows that the last shell of titanium has two electrons and the d-orbital has a total of two electrons3d 2. 67Valence electrons in Holmium Ho13.
The electron configuration of vanadium shows that the last shell of vanadium has two electrons and the d-orbital has a total of three electrons 3d 3. Valence electrons in Neon Ne 8. 15 How many core and valence electrons does s have.
8 How did you calculate the number of electrons of lead Pb. They will have at least one 3d-electron and all except Cr and Cu will have two electrons in the 4s the highest energy sublevel. Valence electrons in Phosphorus.
7 What group number is indium in. 6 What is vanadium valence. 71Valence electrons in Lutetium Lu3.
How Many Valence Electrons In Vanadium5 valence electronsHow many valence electrons are present in vanadiumfive valence electronsVanadium has five valence electronsDoes vanadium have 5 valence electronsThe electron configuration of vanadium shows that the last shell of vanadium has two4s2 ele. Elements may steal an electron from the outermost s block and relocate it to the d block in order to reach a filled or half-filled 5 of 10 electrons state in the d block. 4 How do you find valence electrons.
18 Can sulfur have more than 8 valence electrons. 8 What is the valence of fluorine. Most transition metals have 2 valence electron.
13 Why is vanadium 5 valency. Consider the first-row transition metals d-block Sc - Zn. For example the total number of valence electrons in oxygen dichloride OCl2 is given by.
4 How many electrons are in the outermost shell of indium. Valence electron are the sum total of all the electrons in the highest energy level principal quantum number n. The electron configuration of manganese shows that the last shell of manganese has two electrons and the d-orbital has a total of five electrons3d 5.
12 What group is. 66Valence electrons in Dysprosium Dy12.

Determining Ionic Charges And Valence Electrons Electrons Ionic Transition Metal

How To Find The Number Of Valence Electrons Using A Periodic Table Electrons Energy Level Chemistry
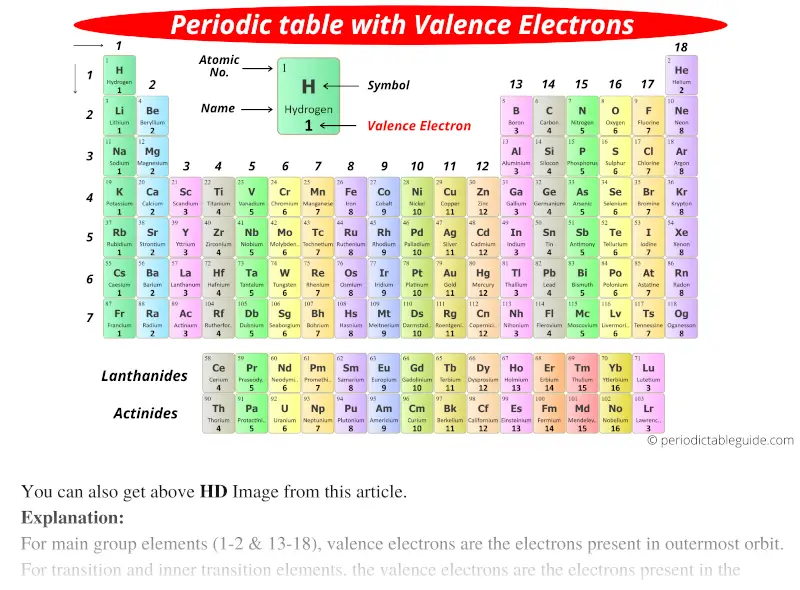
Periodic Table With Valence Electrons Labeled 7 Hd Images

How To Find The Number Of Valence Electrons

Electron Configurations And The Periodic Table Electron Configuration Periodic Table High School Chemistry

What Are Valence Electrons Chemtalk

8 3 Electron Configurations How Electrons Occupy Orbitals Chemistry Libretexts Electron Configuration Atomic Structure Aufbau Principle
.PNG)
Valence Electrons Presentation Chemistry

Valence Electrons Video Khan Academy

Lesson Explainer Electronic Configurations Of Transition Metals Nagwa

How To Find The Number Of Valence Electrons For Transition Metals Youtube
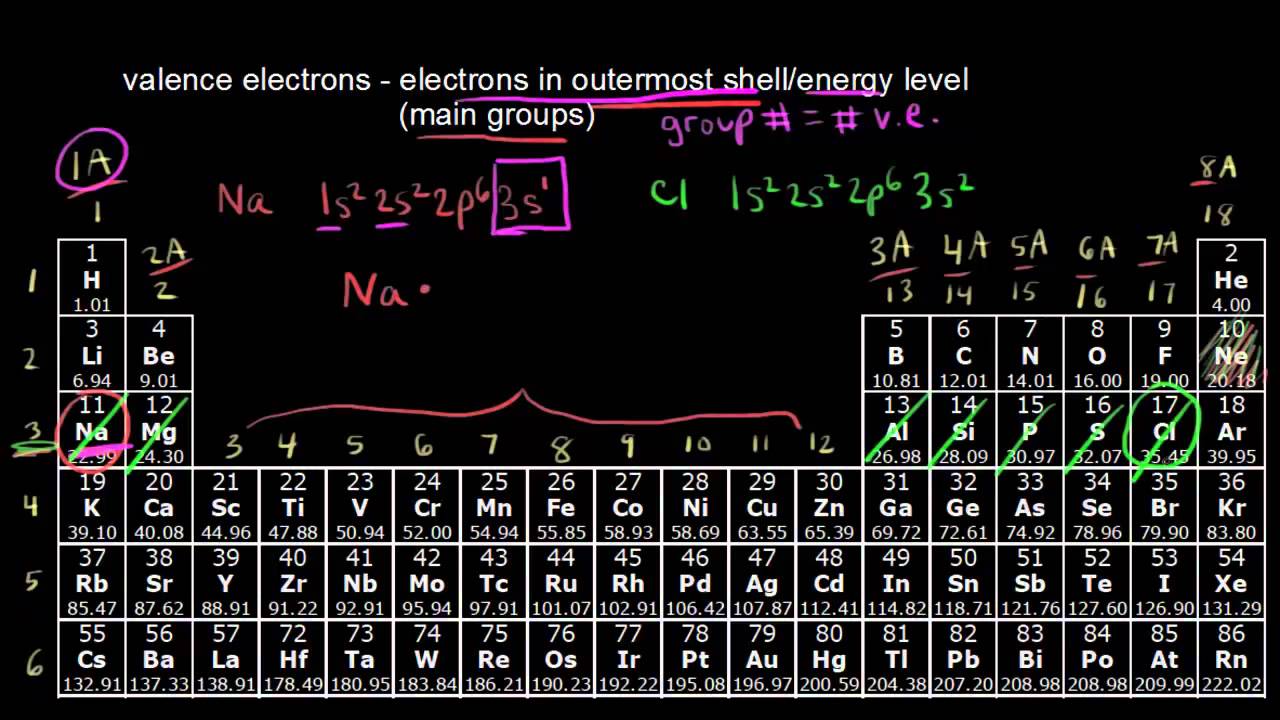
Counting Valence Electrons For Main Group Elements Video Khan Academy

Electron Configuration Chemwiki Electron Configuration Electrons Transition Metals Chemistry

Transition Metals Elements Definition List Properties Transition Metal Electron Configuration Ionization Energy

How To Find Valence Electrons Chemistry Classroom Science Chemistry Chemistry

Valency Periodic Table Online Science Science

We Are Having A Tech Free Day And Thought Between Transition Metal Bingo And Periodic Physical Science Middle School Middle School Science Chemistry Education

Comments
Post a Comment